We develop state-of-the-art materials and technologies for solving biomedical problems. Examples include conductive materials with the capability of utilization as sensor and actuator in human body as well as scaffolds that aid in engineering of cardiac tissues and their differentiation from stem cells.
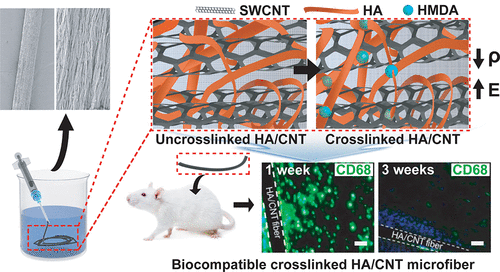
Biocompatible Carbon Nanotube-based Hybrid Microfiber for Implantable Electrochemical Actuator and Flexible Electronic Applications
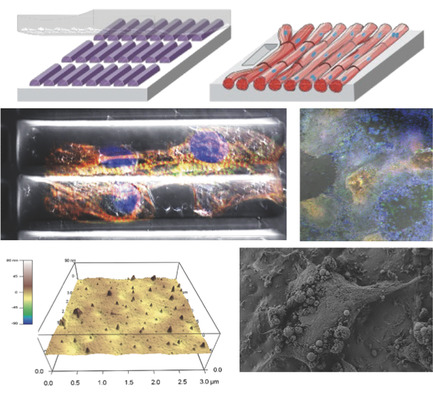
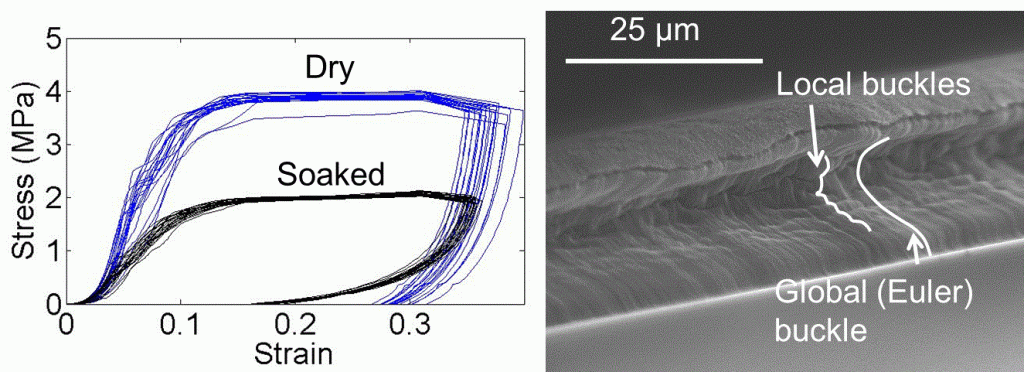
Biocompatible, electrically conductive microfibers with superior mechanical properties have received great attention due to their potential applications in various biomedical applications such as implantable medical devices, biosensors, artificial muscles, and microactuators. Here, we developed an electrically conductive and mechanically stable carbon nanotube-based microactuator with low degradability that makes it usable for an implantable device in the body or biological environments. The microfiber was composed of hyaluronic acid (HA) hydrogel and single-wall carbon nanotubes (SWCNTs) (HA/SWCNT). HA hydrogel acts as bio-surfactant and ion-conducting binder to improve the dispersion of SWCNTs resulting in enhanced electrical and mechanical properties of the hybrid microfiber. In addition, HA was crosslinked to prevent leaking of the nanotubes from the composite. Crosslinking of HA hydrogel significantly enhances Young’s modulus, failure strain, toughness, stability of electrical conductivity, and resistance to degradability and creep of hybrid microfibers. The obtained crosslinked HA/SWCNT hybrid microfibers show excellent capacitance, and actuation behavior under mechanical loading with the low potential of ±1 V in a biological environment. Furthermore, the HA/SWCNT microfibers exhibits excellent in vitro viability. In vivo biocompatibility is also shown through the resolution of early inflammatory response in less than three weeks after implantation of the microfibers in subcutaneous tissue of mice.
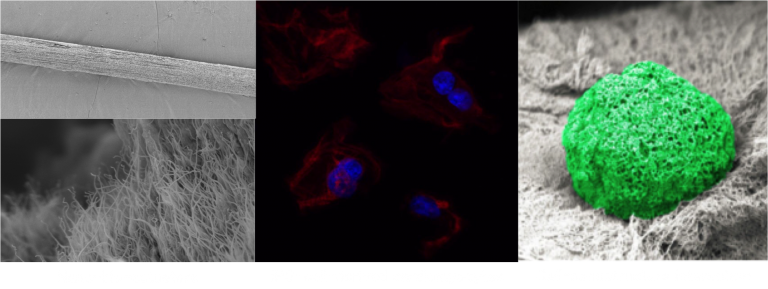
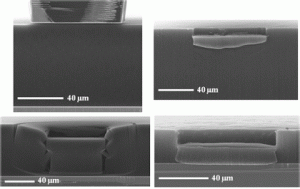
Engineering of Mature Human Induced Pluripotent Stem Cell‐Derived Cardiomyocytes Using Substrates with Multiscale Topography
Producing mature and functional cardiomyocytes (CMs) by in vitro differentiation of induced pluripotent stem cells (iPSCs) using only biochemical cues is challenging. To mimic the biophysical and biomechanical complexity of the native in vivo environment during the differentiation and maturation process, polydimethylsiloxane substrates with 3D topography at the micrometer and sub‐micrometer levels are developed and used as cell‐culture substrates. The results show that while cylindrical patterns on the substrates resembling mature CMs enhance the maturation of iPSC‐derived CMs, sub‐micrometer‐level topographical features derived by imprinting primary human CMs further accelerate both the differentiation and maturation processes. The resulting CMs exhibit a more‐mature phenotype than control groups—as confirmed by quantitative polymerase chain reaction, flow cytometry, and the magnitude of beating signals—and possess the shape and orientation of mature CMs in human myocardium—as revealed by fluorescence microscopy, Ca2+ flow direction, and mitochondrial distribution. The experiments, combined with a virtual cell model, show that the physico‐mechanical cues generated by these 3D‐patterned substrates improve the phenotype of the CMs via the reorganization of the cytoskeletal network and the regulation of chromatin conformation.